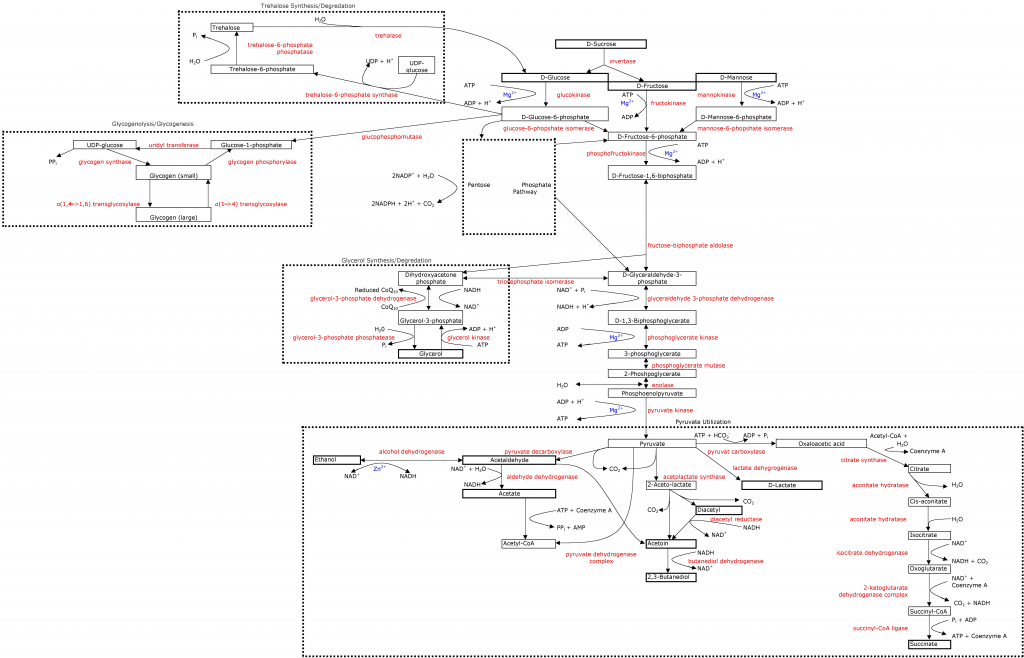
The end goal: understanding exactly what happens to sugars that are transported into the cell, and what byproducts result from these metabolic pathways, and their significance to the final product whether it be wine, mead or beer. You should be able to understand, and navigate this superpathway map by the end of these:
For this discussion we will focus on just Saccharomyces Cerevisiae, the main microbe used for alcoholic fermentations. Note that many other microbes will use similar processes, either with different end products, or different intermediates, depending on the species and the environment. For example, lactobacillus species will favor the metabolism pyruvate into lactic acid as opposed to ethanol, and brettanomyces species will produce far more acetate (via the PDH bypass route) in the presence of oxygen than saccharomyces species.
Preliminaries
In order to make this discussion beneficial, it is essential to have some basic understanding of chemistry and it's terminology. If you already have an understanding of basic organic chemistry and biology, feel free to skip to the next section.
Organic - does not mean no chemicals! The names of many compounds makes them seem vary dangerous, but be aware that all the compounds we will discuss occur naturally (either synthesized by other organisms, or alone in nature). In chemistry, organic just means that the compounds contains carbon, and inorganic means no carbon. This means that water is inorganic from a chemical standpoint.
Sugars - end in -ose. They can be classified very broadly by the number of carbon atoms in the molecule, typically using greek as the base. So a six carbon sugar would be a hex-ose; five, a pent-ose; three, a tri-ose.
Ions and salts - a salt (in chemical terms) is a compound formed by an ionic bond. The first (the cation) has a positive charge because it has lost at least one (possibly more) electrons from one of its atoms (or several atoms); the second (the anion) has a negative charge due to a gain of an electron(s). This process results in charged molecules that bind together (via the same process as magnets) very strongly. Salts tend to separate in solution to form free ions that can react with other ions. Negatively charged ions (anions) tend to have the suffix -ate (they can also have the suffix -ite, or prefix hypo- or hyper-, depending on the specific ions charge compared to its normal state).
Acids - the definition we are concerned about is that acids donate protons (H+ ions). Many acids are in fact ionic salts who's cation is H+, but there are many organic compounds that do not form ionic bonds with hydrogen ions, but still donate protons when in solution making them acids. The left over anion tends to end in -ate (ie. acetic acid → H+ + acetate).
Enzymes - end in ase. They are complex proteins that catalyze reactions, making them fast enough to allow the vital functions of life; with out them, these reactions would take too long to form the complex metabolic functions required for most life. The input (or initial compound) is called the substrate, and the end result (compound after transformation) is called the product. Many enzymes exist in very large complexes made of several variants of a single enzyme, or related enzymes in the same "family".
Cofactors - most enzymes require other compounds to help with their functions called cofactors. These can be ions, or complex organic compounds, many of which are vitamins. Enzyme complexes utilize coenzymes, to help with multi step reactions, which can be considered cofactors.
Numbers - can mean a few different things in chemistry. A superscript number (accompanied by a +/- sign) tells the charge of an ion/molecule compared to 0. A subscript represents how many of that specific atom there are (CO2 has 2 oxygen atoms). A number in the name of a compound (separated by hyphens) represents a specific atom within the compound that the following ion/compound is attached to (glucose-6-phosphate has a phosphate group on the 6th carbon atom in a glucose molecule; fructose-1,6-biphosphate has a phosphate group on the 1st and 6th carbon atoms of a fructose molecule).
Oxidation/Reduction (redox) - in a strictly chemical sense, oxidation means that a compound/atom lost an electron. The opposite reaction, a compound/atom gaining an electron, is called reduction. These reactions are incredibly important as the ratio of oxidized vs reduced compounds drives what reactions take place in a cell. The cell diverts metabolic intermediates to different metabolic pathways to create imbalances in the redox state, forcing the cell to use certain other pathways to rebalance the redox state within the cell.
The yeast cell itself has many different component parts, but we are only concerned with a few for this discussion. The cell wall, which allows transport of molecules into and out of the cell, is composed of mostly β-1,3-glucan and mannoproteins (about 50% and 40% respectively, the rest being about 10% β-1,6-glucan and small amounts of chitin). The inside of the cell is full of a liquid called cytosol, which is comprised of about 70% water, the rest being comprised of many ions, proteins and enzymes. The cytosol is where the vast majority of the metabolic pathways about to be discussed occur. The other cellular component we are interested in is the mitochondria, which serves as the "power plant" of the cell of most organisms. While it's role is critical for yeast, because yeast do not regularly use aerobic respiration, this "power house" aspect of the mitochondria is not wholly relevant. What is relevant is the yeasts' use of the TCA cycle enzymes (also called the citric acid or Krebb's cycle), though in a diminished capacity compared to other organisms.
Organic - does not mean no chemicals! The names of many compounds makes them seem vary dangerous, but be aware that all the compounds we will discuss occur naturally (either synthesized by other organisms, or alone in nature). In chemistry, organic just means that the compounds contains carbon, and inorganic means no carbon. This means that water is inorganic from a chemical standpoint.
Sugars - end in -ose. They can be classified very broadly by the number of carbon atoms in the molecule, typically using greek as the base. So a six carbon sugar would be a hex-ose; five, a pent-ose; three, a tri-ose.
Ions and salts - a salt (in chemical terms) is a compound formed by an ionic bond. The first (the cation) has a positive charge because it has lost at least one (possibly more) electrons from one of its atoms (or several atoms); the second (the anion) has a negative charge due to a gain of an electron(s). This process results in charged molecules that bind together (via the same process as magnets) very strongly. Salts tend to separate in solution to form free ions that can react with other ions. Negatively charged ions (anions) tend to have the suffix -ate (they can also have the suffix -ite, or prefix hypo- or hyper-, depending on the specific ions charge compared to its normal state).
Acids - the definition we are concerned about is that acids donate protons (H+ ions). Many acids are in fact ionic salts who's cation is H+, but there are many organic compounds that do not form ionic bonds with hydrogen ions, but still donate protons when in solution making them acids. The left over anion tends to end in -ate (ie. acetic acid → H+ + acetate).
Enzymes - end in ase. They are complex proteins that catalyze reactions, making them fast enough to allow the vital functions of life; with out them, these reactions would take too long to form the complex metabolic functions required for most life. The input (or initial compound) is called the substrate, and the end result (compound after transformation) is called the product. Many enzymes exist in very large complexes made of several variants of a single enzyme, or related enzymes in the same "family".
Cofactors - most enzymes require other compounds to help with their functions called cofactors. These can be ions, or complex organic compounds, many of which are vitamins. Enzyme complexes utilize coenzymes, to help with multi step reactions, which can be considered cofactors.
Numbers - can mean a few different things in chemistry. A superscript number (accompanied by a +/- sign) tells the charge of an ion/molecule compared to 0. A subscript represents how many of that specific atom there are (CO2 has 2 oxygen atoms). A number in the name of a compound (separated by hyphens) represents a specific atom within the compound that the following ion/compound is attached to (glucose-6-phosphate has a phosphate group on the 6th carbon atom in a glucose molecule; fructose-1,6-biphosphate has a phosphate group on the 1st and 6th carbon atoms of a fructose molecule).
Oxidation/Reduction (redox) - in a strictly chemical sense, oxidation means that a compound/atom lost an electron. The opposite reaction, a compound/atom gaining an electron, is called reduction. These reactions are incredibly important as the ratio of oxidized vs reduced compounds drives what reactions take place in a cell. The cell diverts metabolic intermediates to different metabolic pathways to create imbalances in the redox state, forcing the cell to use certain other pathways to rebalance the redox state within the cell.
The yeast cell itself has many different component parts, but we are only concerned with a few for this discussion. The cell wall, which allows transport of molecules into and out of the cell, is composed of mostly β-1,3-glucan and mannoproteins (about 50% and 40% respectively, the rest being about 10% β-1,6-glucan and small amounts of chitin). The inside of the cell is full of a liquid called cytosol, which is comprised of about 70% water, the rest being comprised of many ions, proteins and enzymes. The cytosol is where the vast majority of the metabolic pathways about to be discussed occur. The other cellular component we are interested in is the mitochondria, which serves as the "power plant" of the cell of most organisms. While it's role is critical for yeast, because yeast do not regularly use aerobic respiration, this "power house" aspect of the mitochondria is not wholly relevant. What is relevant is the yeasts' use of the TCA cycle enzymes (also called the citric acid or Krebb's cycle), though in a diminished capacity compared to other organisms.
The form of energy used, and released from metabolic functions is typically stored in two families of compounds: adenosine mono/di/tri-phosphates (AMP, ADP, and ATP) which transport energy in the form of phosphate (PO4-) groups (and their bonds), and Nicotinamide adenine dinucleotide and it's reduced partner (NAD+ and NADH respectively) which allows transport of reductive/oxidative energy (electrons). Nicotinamide adenine dinucleotide phosphate, and the reduced form (NADP+ and NADPH), also serves the same purpose in certain metabolic pathways. Each step has a very specific group of enzymes that catalyze the reaction, and certain steps also require other ions or molecules (cofactors) that are bound to enzymes or energy molecules (ie. ADPMg-, or ATPMg2-)
Glycolysis
Almost all organisms have evolved to use glycolysis as a means of energy generation, and it is crucial to understand this process if you want to know what is happening in a fermenting must/wort. In it's simplest terms, glycolysis is the transformation of glucose* to pyruvate**, which in turn can be transformed into ethanol, acetyl-CoA, or other compounds depending on the cells needs, and the organism's preferred processes.
Glycolysis is normally broken up into two phases: investment (or preparatory) and reward (or pay-off). In the investment phase, two phosphate groups are taken from two ATP molecules creating two ADP molecules (which contain less energy), and glucose is split into two D-glyceraldehyde 3-phosphate (triose sugars that will be turned into pyruvate in the second phase of glycolysis). The equation for the first part of glycolysis is:
C6H12O6 + 2ATP → 2C3H7O6P + 2ADP + 2H+
(the hydrogen and oxygen atoms are conserved via the nature of the phosphate groups in ATP vs ADP).
The first step in the preparatory/investment phase is the phosphorylation of D-glucose by a family of enzymes called hexokinases. This step breaks one phosphate group (Pi) off of ATP (necessarily bound to a magnesium ion) and adds it to the sixth carbon in glucose molecules, creating α-D-glucose-6-phosphate (G6P), ADP and a free hydrogen ion. This step is crucial for maintaining the cells preferred osmotic pressure by lowering the external glucose concentration; furthermore, G6P cannot be transported through the cell wall so it must continue on the process once started.
The second step is the isomerization (rearrangement) of α-D-glucose-6-phosphate into β-D-fructose-6-phosphate (F6P) using the enzyme phosphoglucose isomerase. This process is freely reversible; momentum is kept moving forward due to the normally low concentration of F6P, but in high fructose environments, it can run in reverse. At this point there are two ways in which yeast can get F6P: either by isomerization of glucose-6-phosphate, or from fructose that has been phosphorylated via the enzyme fructokinase (a hexokinase). Some yeast will readily use fructose (those deemed "fructophile" - liking fructose), whereas many will prefer to work with glucose (called "glucophilic" yeasts), only to later use the fructose, which often results in residual fructose (this is often considered a fault in wine as fructose is perceived to be much sweeter than glucose, leaving a wine "too sweet").
The third step of glycolysis is the phosphorylation of β-D-fructose-6-phosphate to β-D-fructose-1,6-biphosphate (F1,6BP) via the enzyme 6-phosphofructokinase. This process cleaves a phosphate group off of ATP (bound to a magnesium ion) and results in ADP and a free H+ ion, and represents the last expenditure of ATP in the process. This is also the point of no return for the process so far, F1,6BP has to continue through the process in order to be beneficial to the yeast.
The extra phosphate group results in the destabilization of the molecule which allows the creation of two charged molecules in the steps that follow, not allowing the compounds to leave the cell. This is also one of the steps where the cell can limit the rate of glycolysis (the other two are step one and ten) by limiting the concentration of the phosphofructokinase enzyme.
The fourth step is the splitting of β-D-fructose-1,6-biphosphate by the enzyme fructose-biphosphate aldolase resulting in two different molecules: D-glyceraldehyde-3-phosphate (GADP) and dihydroxyacetone phosphate (DHAP). It is important to note that yeast use a class II aldolase which typically uses a transition metal ion (normally zinc) as a cofactor, whereas animals and plants use a class I aldolase.
The next step is almost instantaneous. An enzyme called triosephosphate isomerase rapidly converts DHAP to GADP and vice versa. As GADP is the molecule that continues with the process of glycolysis, this enzyme allows the same process to be used on both molecules from the previous step.
This ends the investment/preparatory phase in which a single molecule of glucose (or fructose) has been split into two triose molecules. The following reactions all take place in duplicate for a total formula of:
The sixth step of glycolysis takes the D-glyceraldehyde 3-phosphate produced in the last step and makes D-1,3-biphosphoglycerate using a phosphate group (usually HPO4), NAD and an H+ ion.
The seventh step results in the first generation of ATP by cleaving a phosphate group off of D-1,3-biphosphoglycerate, via the enzyme phosphoglycerate kinase, and adding it to a ADP molecule, leaving 3-phosphoglycerate. As this reaction happens two times for each molecule of glucose consumed, this step creates 2 ATP molecules, repaying the two used in the investment phase (net gain = 0). As this step utilizes ATP/ADP, magnesium is a cofactor.
Step eight is a simple isomerization of 3-phosphoglycerate to 2-phosphoglycerate, using phosphoglycerate mutase.
Step nine transforms 2-phosphoglycerate into phosphoenolpyruvate using the enzyme enolase. This reaction requires 2 magnesium ions (one associated with the carboxylate group of the substrate, the other as a catalyst for dehydration).
The final step in glycolysis is the cleaving of the phosphate group off of phosphoenolpyruvate to generate ATP (2 per each molecule of glucose that enters glycolysis; net gain = 2), and pyruvate by the enzyme pyruvate kinase. This step requires a magnesium ion (bound to ADP) and a hydrogen ion.
Once pyruvate is created, the cell will shunt it through different metabolic pathways to meat the cells needs given it's present state. This entire process, glycolysis, has several branch points where certain intermediates can follow other metabolic pathways, creating many more compounds than just ethanol. These branch points will be discussed in following articles.
Takeaways
- Yeast can use glucose or fructose as an energy source by converting it to different compounds and "capturing" the released energy for other uses
- These processes all require enzymes, which are made out of amino acids, showing how important amino acids truly are
- Lots of phosphate groups are used throughout this and other biochemical processes, showing that yeast need some form of PO4- for healthy metabolism, whether it's from DAP or organic sources
- Magnesium is also important for yeast (and other organisms') metabolism due to it's use throughout the process
- Zinc is needed at a very critical point in glycolysis, and we will see its pivotal role in ethanol fermentation as well, making proper levels very important for yeast health
- From a single hexose, two pyruvate molecules, and a net gain of two ATP molecules, can be produced, making this process very energy efficient
- Most importantly, glycolysis is only a single pathway that molecules can follow that branches out into many other pathways and fuels almost every action the cell can make; while its individual importance can not be underestimated, it is the holistic understanding of all the branches, and where they lead, that truly matters.
* greek γλεύκος - must, sweet wine; related greek γλυκύς- sweet; the upsilon is written υ/Υ which explains the older term glycose for glucose
** greek πύρ - fire and latin uva - grape; named because it was discovered by the dry distillation of racemic acid (derived from grapes); again note the υ/Υ switch yielding pyruvate.










No comments:
Post a Comment