This will be a series of articles that compare the constituent compositions of grape musts and honey musts, which is worse than comparing apples and oranges. Although we try to use similar practices on both for the production of wines and meads, there are intrinsic differences that can cause problems; but that also make each unique, even down to the specific grape variety or nectar source. These will not be all encompassing, comparing Russian River Pinot to Burgundy, or Arizona orange blossom to Floridian, but will serve as a guide to how we should treat our musts: what to add, when to add it, and what not to add it.
First we are going to take a look at the minerals in wine and mead. Why? Brewers worry night and day about their water, and for good reason; winemakers almost never consider it because whatever is in the grapes is considered good enough (most of the time); but mead makers don't tend to consider it at all, even though we are adding considerable amounts of it to our honey. As it stand now, no one has done a comparison of different water salt additions to test the organoleptic quality imparted by them, something I hope to do when I bottle some of last years traditionals. Until such time, all we can do is compare the minerals in mead musts to those of wine musts and try to guess what is going on and what needs to be corrected.
So, what specific minerals are we interested in? There are actually two questions there:

- What minerals do yeast need for healthy growth?
- What minerals have an impact on the flavor of the product?
The first is readily available thanks to decades of extensive research on yeast metabolism and preferred growth media. The second is very hard in this context: I have yet to find any published papers on the effect of specific minerals on the quality of wine, let alone mead. Aside from obscure remarks about vineyard salt levels and the quality of grapes from them, it seems that no one is putting in any research on the organoleptic side. This is an area where brewers run circles around winemakers, and it is rather strange considering all the hype over the "minerality"of certain wines, although anyone who says they can taste slate is an idiot (go ahead, lick a piece of slate, then granite, and then quartz, they all taste the same; do it blindfolded if you want!).
To the right is a chart comparing the levels of common ions in wine musts, honey, and mead musts, as well as a common synthetic must that is used in experiments. From it, we can see just how different wine must, mead must, and what scientists think wine must is, all are. Wine musts have far greater levels of potassium (K
+), magnesium (Mg
2+), zinc (Zn
2+), sulfate (SO
42-), phosphate (PO
43-), in fact, all the minerals listed are in higher concentration in wine musts than mead musts. This is just further proof that honey is a very inhospitable environment for most organisms.
So, the question remains: do we need to adjust these levels, or is grape must just in excess? For that, we need to look at several very important ions, and a few ratios, and ask they yeast what they want, what they need, and what they prefer.
Potassium (K+)

In brewing, potassium is not considered in a water profile because malt provides the amounts needed for yeast, but in wine and mead it is a very important consideration. Potassium plays a few vital roles concerning the yeast cell; first and foremost, yeast will uptake potassium ions in exchange for hydrogen ions in order to balance the pH in the cytoplasm. H
+ is produced in several steps of glycolysis, and if allowed to remain in the cell, the pH will drop to fast and cause many problems with the glycolytic pathway (and several other pathways). The second role potassium plays is to increase glucose uptake rates via an unknown process that probably occurs with the yeast's hexose transport pathways.
How much potassium is needed? It depends on the amount of H
+ ions within the medium. It has been shown that yeast need a molar ratio of 25-30:1 K:H in order to successfully complete a fermentation. If the ratio falls below this level, the fermentation is prone to sticking early, leaving a considerable amount of residual sugar. The amount of potassium needed can easily be calculated utilizing the pH of the must as seen to the right, but this calculation can be complicated when concerning mead due to the low pH buffering of meads. I'd suggest estimating how much you need based on the pH when the must is first created (before any additions are made), and just use that. To simplify matters, an addition of 0.25g/L (~1g/gal) potassium carbonate will yield 141ppm K, adding this value to the average 237ppm K in a SG 1.108 honey must gives 378ppm K, enough for a pH above 3.45. 1g/L cream of tartar yields 208ppm K, and 0.25g/L potassium carbonate and 1g/L creamo of tartar added together will give 349ppm K, enough to supplement even the lowest K levels found in honey.
It is also important to note that supplying K later in the fermentation does not correct an initial deficit, so there is no point in waiting to add potassium.
Calcium (Ca2+)
Considered important by brewers because it stimulates yeast flocculation (and helps control mash pH), calcium is not terribly important in wine or mead making as the bulk aging time will easily clear yeast from the product. In fact, calcium acts antagonistically to magnesium and zinc uptake, and while small amounts (~1ppm) are needed for cell wall maintenance, it's use should be limited to low levels if any is added at all (be aware that almost all nutrient blends contain calcium as a cation attached to certain vitamins, and they usually contain magnesium to help balance this out). Also note that bentonite additions will add calcium to the product if used as a fining agent.
Magnesium (Mg2+)
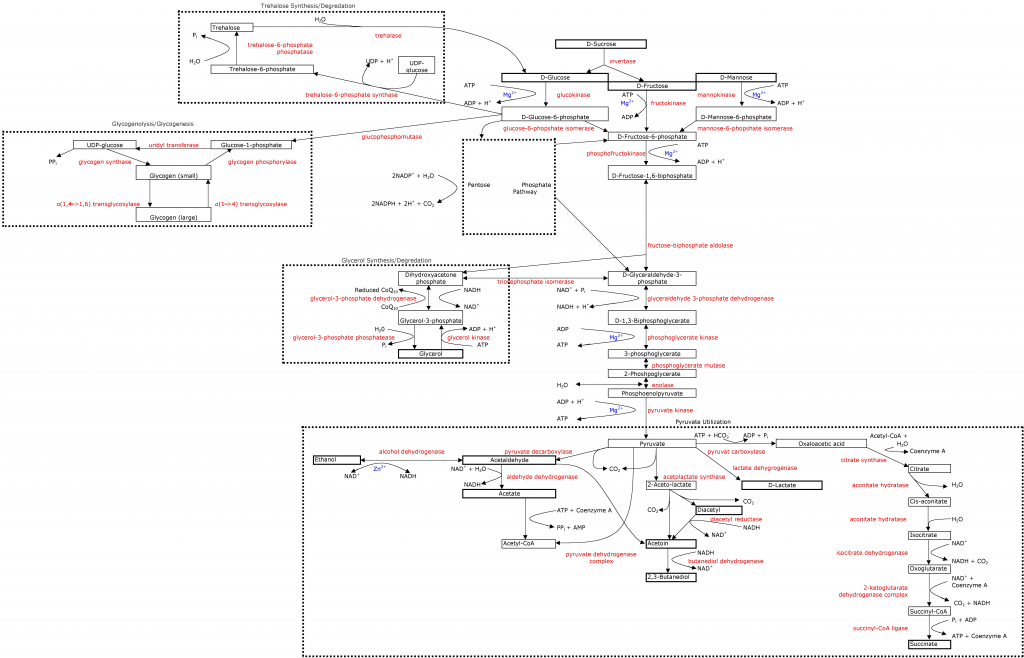
As seen in the
glycolytic pathway, yeast need magnesium for almost all cellular function, especially glycolysis. This is another mineral that brewers don't have to worry about because malt provides a ton of it; and winemakers tend to have enough of it, they just need to worry about if they have a proper amount compared to their calcium content. What is the proper amount? Something higher than 1:1 Mg:Ca. Many studies have been done showing that yeast function better with higher Mg:Ca ratios (2-4:1), but this may be excessive. I think a 1.5-2:1 ratio is right about where we should be to optimize yeast performance and not risk too rapid growth, zinc interference, or off flavors.
Sodium (Na+)
Have you ever noticed that Australian wines tend to be really savory, almost salty? This mineral is responsible for a lot of that flavor. Yeast don't need it, but it is always amazing how it can bring that cup of broth up to the next level, if used carefully. Brewers concern themselves with the chloride ion to give round, mouth-filling, savoriness to a beer, and some add a lot of salt (NaCl); others would never touch it and just use calcium chloride; but there is a difference between adjusting chloride levels, and adding sodium, and in moderation sodium just gives things that extra bit of something. How very scientific of me!
Chloride (Cl-)
Chloride, just like sodium, has no important function in yeast metabolism, and is an unknown as far as flavor contributions to mead. The levels found in grape musts are obviously higher than those in mead, but most commercial nutrient blend provide small amounts in the form of thiamine hydrochloride and other vitamin or amino acid based salts.
Sulfate (SO42-)
Sulfates are very important for yeast health as yeast need two sulfur containing amino acids (cysteine and methionine) which are often synthesized from other amino acid skeletons and sulfate via the sulfate reduction pathway. It is very important to note that this pathway relies on sulfate (SO
42-) and not sulfite (SO
32-) or elemental sulfur (S), of which the latter can lead to increased hydrogen sulfide (H
2S) production.
Sulfate is generally added as an anion attached to another mineral that we choose to add (ie. MgSO
4, magnesium sulfate). The addition of sulfate may have an affect on the flavor profile of wine and mead, though it is unclear what the impact is.
Phosphate (PO43-)
Phosphates are very important for yeast metabolism (see how many times they occur just in glycolysis), allowing the construction of nucleic acids, energy transferring compounds (ATP), cell membranes and other internal structures, and are vital for the transport of extracellular compounds into the cell. Simply put, yeast needs phosphate, and honey does not have enough! The levels found in wine are on average 20-30 times those found in mead musts. Luckily, the P in DAP (diammonium phosphate) is there to help out; this compound is put in all non organic nutrient blends to provide both easy nitrogen, in the form of ammonium, and phosphates. A simple 0.25g/L addition of DAP will give 180ppm phosphate which is more than enough for yeast health. Note, however, that the 53ppm yeast assimilable nitrogen (YAN) provided by this same addition is normally not enough for mead musts; in order to get 212ppm YAN form just DAP, an addition of 1g/L is needed, which results in 719ppm phosphate (almost double the levels found in the highest testing wine samples, and above the legal limit of some countries).

Trace Minerals
Small amounts of manganese (Mn), copper (Cu), iron (Fe), molybdenum (Mo), boron (B), zinc (Zn), cadmium (Cd) and even lead (Pb) are present in grape musts with the table to the left representing an "average" Portuguese white wine must. Manganese tends to run at about 0.5-7.3ppm (mean of 2.7ppm) in most european wines, and zinc is be all over the board from 0.1-10ppm (with some chinese musts registering >12ppm). Typically lead is below 1ppm, as well as copper and iron. Almost all the same trace minerals can be found in honey, but in much lower quantities, with almost no copper or iron.
Fermentation rates seem to be best when there is about 2ppm Zn, 11ppm Mn, and 15ppm Fe, however those concentrations of manganese and iron would clearly impact flavor, and as they are not essential (above trace amounts), there is no need to supplement musts with them. Zinc, however, can have a major impact on the progress of fermentation and should be supplemented to sufficient levels (servomyces works very well at the recommended rate of 8.5ppm (0.32g/gal)).
Takeaways
- Add potassium, even if you don't have a way to measure pH, your must is probably lacking
- If you can measure pH, use the chart to guess how much you need, keeping in mind that any addition will raise the pH
- We don't know what flavor is added or changed, by what ion, yet
- Don't worry about calcium, maybe try to get it to >50ppm if you want, but make sure you add enough magnesium
- Add enough magnesium to get to about 100ppm, or about 1.5 times the calcium; remember that nutrient blends usually have it so don't go too crazy, maybe 2g/gal epsom salt
- Sulfates are needed, but are usually provided by nutrient blends or added epsom salt
- Phosphates are covered by DAP additions
- Trace minerals are present, but zinc may need to be added
- Lighter honeys have less minerals than dark honeys, but not by a lot
Birch, Rosslyn M., Maurizio Ciani, and Graeme M. Walker. "Magnesium, Calcium and Fermentative Metabolism in Wine Yeasts." Journal of Wine Research 14.1 (2003): 3-15. Taylor & Francis Online. 04 Aug. 2010. Web.
Kudo, Masayoshi, Paola Vagnoli, and Linda F. Bisson. "Imbalance of PH and Potassium Concentration as a Cause of Stuck Fermentations." American Journal of Enology and Viticulture 49.3 (1998): 295-301. Print.
Larcher, Robert, and Giorgio Nicolini. "Elements and Inorganic Anions in Winemaking: Analysis and Applications." Hyphenated Techniques in Grape and Wine Chemistry. Ed. Riccardo Flamini. Chichester, England: John Wiley, 2008. N. pag. Print.
Ribéreau-Gayon, P., Y. Glories, A. Maujean, and Denis Dubourdieu. Handbook of Enology the Chemistry of Wine: Stabilization and Treatments. Chichester: John Wiley, 2006. Print.
Ricardo-da Silva, George/Jane M., H. Mira, P. Leite, and A. S. Curvelo-Garcia. "Metal Reduction in Wine Using PVI-PVP Copolymer and Its Effects on Chemical and Sensory Characters." Vitis -Geilweilerhof 46.3 (2007): 138-47. Print.
Somda, Marius K., Aly Savadogo, Nicolas Barro, Philippe Thomart, and Alfred S. Traore. "Effect of Minerals Salts in Fermentation Process Using Mango Residues as Carbon Source for Bioethanol Production." Asian Journal of Industrial Engineering 3.1 (2011): 29-38. Science Alert. 29 July 2011. Web.
Stobbaerts, R., H. Robberecht, F. Haesen, and H. Deelstra. "Manganese Content of European Wines." International Journal of Vitamin and Nutritional Research 64.3 (1994): 233-36. Print.